Two Distinct Trials Share a Same Ethics Approval
Trials registered by a same research group at The First Affiliated Hospital of Anhui Medical University (安徽医科大学) are found sharing a same ethics approval.
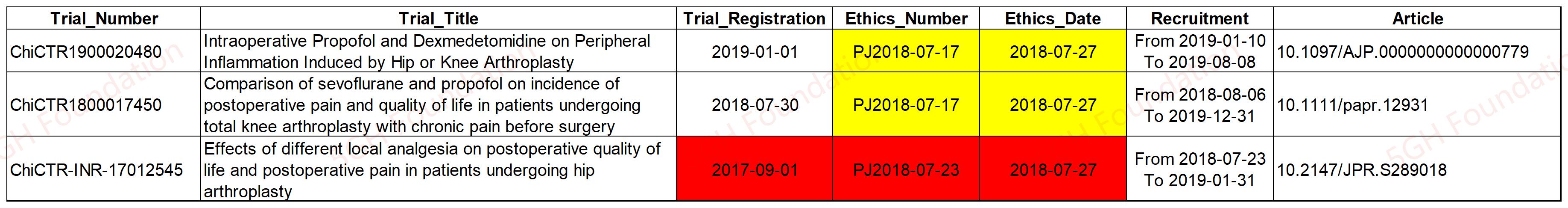
While the intervention of the two trials ChiCTR1900020480 (propofol and dexmedetomidine) and ChiCTR1800017450 (sevoflurane and propofol) were different to each other, the information on the "Chinese Clinical Trial Registry" (ChiCRT) shows that they shared a same ethics approval (with the number of PJ2018-07-17) from the hospital.
This practice was prohibited under under the Chinese regulations. It remains unknown why this occurred. One possible explanation is that the researchers misused the ethics approval numbers when they registered the trials. The ChiCRT should have verified the supporting documents associated with these ethics approvals, but this appears not to have been done.
However, it is hard to rule out the possibility that those ethics approvals were made up by the researcher group. Copies of the ethics approval documents are also required to be posted on the webpages of these trials, however, these documents are not available on the webpages of the two relevant trials.
The 5GH Team also notes that another trial registered by the same researcher group has problematic ethics approval irregularities. The ChiCRT webpage for the trial ChiCTR-INR-17012545 shows that the trial was approved under the number of PJ2018-07-23. However, the ethics approval was granted by the committee at July 27th, 2018, months after the trial was registered (at September 1st, 2017). The recruitment of the participants also began (at July 23th, 2018) days earlier than the ethics approval.
These cases reflect the fact that China has a long history of loose ethics management. Although in recent years, authorities have applied several regulations on this matter, the enforcement is not as strengthened as it is expected.
But the 5GH Team wants to address that not obtianing an approval before the studies is an aspect of this matter, but there are other issues such as abandonments to the protocol, poor animal welfare, unnecessary animal experiments, as well as patients signing the consent without full understanding.
The 5GH Team acknowledges Dr, John Loadsman, who raised the concerns on this research group.